SPOILER ALERT!
Top Caustic Soda Reaction With Chlorine Gas Choices
What is a displacement reaction? Can copper displace iron from iron sulphate answer? Why is phosphorous stored within the water?
Besides family and industrial unintentional exposures, chlorine gasoline has also been used as an agent of war. Germany used chlorine gas in World War I as a chemical weapon. Chlorine gas can also be essentially the most frequent reason for main toxic release incidents internationally. Because of its widespread industrial use, chlorine gasoline has substantial potential for accidental launch. In addition to family exposure, there have been multiple episodes of incidents involving chlorine fuel release.
Sodium bisulfate should also be available as a pH lowering chemical offered alongside the pool chlorinator if hydrochloric acid isn't additionally sold. Be sure to make use of applicable fuel collection equipment to gather the chlorine fuel. Chlorine reacts with KBr, to form potassium chloride, displacing bromine. Since electrical energy is an indispensable uncooked material for the production of chlorine, the vitality consumption corresponding to the electrochemical response cannot be decreased. Energy financial savings come up primarily through applying extra environment friendly technologies and decreasing ancillary vitality use.
Can breathing chlorine make you sick?
Cold Dilute NaOH and Cl2 reaction | NaOH + Cl2 = NaCl + NaOCl + H2O. Cold dilute sodium hydroxide and chlorine gas reaction is disproportionation. Chlorine gas is oxidized and reduced to OCl- ion and Cl- ion respectively. As products sodium chloride, sodium chlorate(i) and water are given.
This occurs as a result of formation of chlorine fuel from NaOCl which is added as a disinfectant. First, chlorine dissolve in water and produce hydrochloric acid (HCl) and hypochlorous acid (HOCl). These two acids react with NaOH individually to offer NaCl and NaOCl respectively. Sodium ion and chloride ion are stable within the water and do not involve in hydrolysis of water. Also, caustic soda slowly reacts with glass and form sodium silicate.
At concentrations of 1 to 3 ppm chlorine gasoline acts as an eye fixed and oral mucous membrane irritant, at 15 ppm there's an onset of pulmonary symptoms, and it can be deadly at 430 ppm inside half-hour. Sometimes hydrochloric acid isn't out there and this may be substituted with an equimolar mixture of sodium chloride salt and sodium bisulfate.
- The caustic exiting the cell line must be monitored for energy, to maintain protected concentrations.
Thus, the upper airway features as a protecting scrubber to the exposure of chlorine gasoline. More severe exposure will induce edema of each the higher airway and the lung parenchyma. Acute lung harm and/or grownup respiratory misery syndrome (ARDS) can also be seen in some severe instances. Chlorine gas is primarily reactive only at a neighborhood level, thus absorbed systemic results are not commonly observed.
What happens when chlorine is passed?
When chlorine is passed over dry slaked lime, bleaching powder will form. equation ---: Ca(OH)2 + Cl2 ---> CaOCl2 + H2O. properties --: it is used as an oxidising agent in many chemical industries. it is used for bleaching cotton , linen in textile industry.
sodium hydroxide lye beads has been used as an agent of war as recently as 2007 in Iraq. Because of its strong odor, chlorine gas can be detected simply. Symptoms of chlorine fuel publicity embrace burning of the conjunctiva, throat, and the bronchial tree. Higher concentrations can produce bronchospasm, lower https://beroilenergy.com/caustic-soda-supplier/ ">caustic soda sds pulmonary damage, and delayed pulmonary edema. Toxicity to chlorine gasoline is determined by the dose and length of exposure.
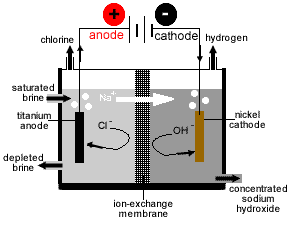
It can be loaded into rail or street tankers via pumps or padded with compressed dry gas. The mercury process is the least energy-environment friendly of the three main technologies (mercury, diaphragm and membrane) and there are additionally concerns about mercury emissions. If sodium piece is kept within the atmosphere, sodium readily rects with oxygen and produce sodium oxide (Na2O).
Produced NaCl and NaOCl are within the resolution and we should always consider, whether or not their ability to hydrolysis of water to make water acidic or basic. There is not any disproportionation reaction sodium hydroxide wholesale oof water and sodium hydroxide. Only sodium hydroxide dissolve very well in water and dissociate to sodium ion and hydroxyl ions completely. naoh sodium hydroxide of brine resolution will produce NaOH, Cl2 gasoline and H2 gasoline.
